
Currently, one of the biggest risks to world health is antimicrobial resistance (AMR). Antimicrobial treatments become less effective as bacteria, viruses, fungi, and parasites become unresponsive to the medicines meant to eradicate them. This poses significant concerns to the health of humans, animals, and the environment. One Health, an all-encompassing and cooperative strategy, is becoming more and more important in the fight against AMR. Antimicrobial stewardship (AMS) with One Health concepts integrated provides a promising way ahead for tackling AMR all at once.
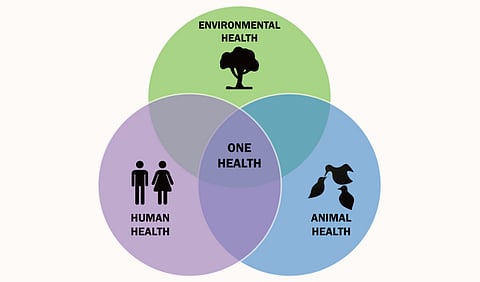
An integrative strategy known as "One Health" places a strong emphasis on the connections between environmental, animal, and human health. It encourages cooperation between different disciplines and sectors to attain the best possible solutions for societal challenges, including AMR. This method acknowledges the tight relationship between human health, animal health, and the ecosystem we all share. The defense against health hazards must therefore be multidisciplinary and coordinated.
AMR is the result of microbes adapting to the therapy used to treat the infections they cause and developing resilience to it. Higher medical expenses, longer hospital stays, and a rise in mortality are caused by this resistance. The overuse and abuse of antimicrobial compounds in agriculture, animals, and people has sped up the emergence of resistant microbes. Since AMR has no geographical boundaries, it is a worldwide issue in need of a coordinated response.
The coordinated effort to maximize the use of antimicrobial drugs is known as "antimicrobial stewardship"(AMS). Enhancing patient outcomes, ensuring cost-effective therapy, and minimizing the negative effects of antimicrobial usage, particularly AMR, are the objectives. Important ideas consist of:
Appropriate use: Making sure antimicrobials are only provided when essential, along with the appropriate dosage and time frame.
Surveillance and Monitoring: Monitoring antimicrobial usage and trends in resistance development.
Education and Awareness: Educating the public and medical professionals on the responsible use of antimicrobials.
Infection Prevention: Putting policies in place to stop infections and the spread of organisms with resistance.
Strategies including the human, animal, and environmental health sectors are necessary for the effective integration of One Health principles into AMS.
1. Collaborative Frameworks: Form multidisciplinary committees and working groups consisting of doctors, veterinarians, scientists, microbiologists, and legislators.
Encourage cross-border cooperation and information exchange to develop unified action plans for AMR.
2. Data Sharing and Surveillance: Create integrated surveillance systems to monitor antimicrobial resistance and usage in the fields of environmental, animal, and human health.
Encourage cross-sector data sharing to spot patterns and create focused actions.
3. Education and Training: Establish educational initiatives to educate the public, farmers, veterinarians, and healthcare professionals about AMR and AMS.
Incorporate One Health concepts into veterinary and medical courses to prepare students for a team-based approach.
4. Policy and Regulation: Improve rules governing the use of antimicrobials in animal husbandry and agriculture, making sure they comply with standards for human health.
Encourage the creation of laws that will aid in the development of novel antimicrobials and alternative therapies.
5. Research and Innovation: Encourage studies on how resistance spreads across animals, people, and the environment.
Make investments in the creation of novel medications, vaccines, and diagnostic instruments that can lessen the need for conventional antimicrobials.
6. Public Engagement: Involve the public in AMR reduction initiatives by highlighting the importance of immunization, good hygiene, and prudent use of antibiotics and other antimicrobial agents.
Employ public opinion initiatives in the media to highlight the significance of a One Health strategy in the fight against AMR.
For this piece, Medbound Times has had the pleasure of having a mindful conversation about the concept of One Health and antimicrobial stewardship with Dr. Linda Oyama, a renowned bio-scientist and an expert in microbiology, antimicrobial resistance, and gut microbiome.
She creates gut microbiome-derived antimicrobials at Queen's University Belfast to treat difficult infections caused by resistant microbes. She is passionate about science outreach and actively advocates for equality through ATHENA SWAN Initiatives, a group that supports women in science.
Dr. Oyama received her PhD from Aberystwyth University, Wales, her MSc from the University of Leeds, England, and her first-class degree in microbiology from Cross River University of Technology, Nigeria. Her work encompasses the discovery of new antimicrobials and the comprehension of their mechanisms, establishing her as a notable figure in biomedical research and teaching.
Can you please tell our readers a bit about your background and how you became involved in the field of One Health and antimicrobial stewardship?
“My name is Linda Oyama, and I am a lecturer at the School of Biological Sciences and Institute for Global Food Security at Queen's University, Belfast. I grew up as a very curious child, and I still am. This curiosity led me towards the sciences. I always wanted to solve problems and found that I particularly enjoyed pursuing something the more challenging I found it. I reasoned that it wouldn’t become boring too quickly if I had to do it every day.
In high school, I ended up focusing on science-related subjects. At the time, the only science careers I knew about were engineering and medicine. In my culture in Nigeria, where I am originally from, medicine is highly regarded. If you were a brilliant science student, you were expected to pursue medicine, and there was little awareness or promotion of other science pathways and careers. Like everyone else, I thought I would become a doctor. However, I didn't get into my first choice for medicine due to various issues with the admissions process, and other factors. As you would expect from a 16-year old, I was very upset but began exploring other science subjects.
I started investigating the university brochures from the Joint Admissions and Matriculation Board (JAMB), which listed all the different science courses and the universities that offered them. I discovered a new university in the state where I had attended secondary school. They offered courses like biochemistry and microbiology, which were affiliated with medical sciences. I decided to choose biochemistry as my first choice and microbiology as my second, even though I didn't know much about either. I figured biochemistry involved biology and chemistry, both of which I liked, and microbiology sounded like biology.
When the admissions list was released, I got into microbiology. Initially, I was disappointed because it wasn't my first choice, but I quickly grew to love the subject. My interest in genetics and medical microbiology was sparked when my little brother was ill and not responding to hospital treatments. I wanted to understand the genes that might be causing his illness. This led me to my first project, where I investigated the antimicrobial properties of local medicinal herbs against drug-resistant hospital infections.
My journey into antimicrobial resistance and chemotherapy began there. Despite my initial disappointment about not studying medicine, I excelled in my field and graduated as the overall best student at the university. I was awarded a scholarship to study anywhere in the world and chose the University of Leeds in the UK for a master’s degree.
By this point, I was tired of lectures, as my undergraduate education in Nigeria, as with many BSc programs, had been very theory-intensive with limited laboratory work. I opted for a research master’s to get a feel for the UK education system and prepare for a PhD. My master’s research focused on understanding how new compounds, like silver ions, worked against the antimicrobial resistant superbug Staphylococcus aureus. This experience was like a mini-PhD, involving a thesis and a viva with an external examiner, which prepared me for the next phase of my journey.
After completing my Master's, I went to Aberystwyth University in Wales for my PhD. There, I expanded my research to metagenomes, studying genetic material from multiple microorganisms for their antimicrobial properties and resistance mechanisms and using machine learning algorithms to identify new antimicrobials from animal gut genetic material and organisms (the microbiome). After my PhD, I worked in Wales, developing novel compounds to treat resistant bacteria.
A few years ago, I moved to Queen's University as a postdoctoral research fellow to continue developing these compounds. At Queen's, I established the Antimicrobial Resistance and One Health Lab (AMR and One Health Lab). In this lab, we develop novel antimicrobials known as antimicrobial peptides and study the origins and transmission of resistance across different ecosystems. The concept of One Health highlights the interconnectedness between humans, animals, and the environment, which is crucial in our modern world.”
What are some key components of One Health that are particularly relevant to antimicrobial stewardship programs?
With One Health, let's start with the environment. Consider the stewardship of how we use herbicides and chemicals on our plants. Not only do these substances kill pests and disease-causing organisms, but they can also accumulate in food, soil, and water reservoirs. Bacteria, fungi, and other microorganisms in these environments quickly develop mechanisms to protect themselves, such as efflux pumps. An efflux pump is a system in bacteria that flushes out harmful substances trying to enter their cells. From this perspective, we must use herbicides and antimicrobials judiciously, understanding the correct amounts and avoiding overuse. It is crucial to comprehend the implications of using these substances, not just for immediate benefits like higher yields and disease prevention but also for long-term impacts on resistance. We need extensive research in this area, as we don't fully understand the long-term effects of some compounds on resistance.
This cautious approach applies to human and veterinary medicine as well. For instance, regulations in the swine industry in Europe and the UK have banned zinc oxide, with the final implementation in June 2024 in the UK. However, enforcing these regulations can be challenging if people perceive benefits from using these substances, despite their potential negative side effects.
In healthcare, patients often demand antibiotics for issues like bad coughs or sore throats without proper checks to see if they are necessary. My spouse, who has an ongoing medical condition and is prone to recurrent bacterial infections, often receives antibiotics in the hospital to prevent worsening infections without proper microbiological tests. However, these antibiotics are sometimes ineffective against the resistant organisms causing the infections, which will inevitably make the organisms even more resistant to future infections.
There are now new tools that can diagnose infections quickly, without the traditional 48- to 72-hour wait. Hospitals and doctors should leverage these tools to ensure accurate diagnoses before prescribing antibiotics. Of course, it is understandable to prescribe urgently while awaiting results, if someone's life depends on immediate treatment, as is sometimes the case. But in less urgent cases, quick diagnostic methods like rapid PCR sequencing or MALDI-TOF mass spectrometry can help. A great example of this is the diagnostic innovation that won the Longitude Prize in AMR recently: a test that can identify the presence of a bacterial infection in a urine sample in just 15 minutes and accurately identify the right antibiotic to treat it within 45 minutes.
In summary, One Health must consider how the use of herbicides in the environment can affect the food chain and water systems. Similarly, misuse of antimicrobials in humans and animals and the shedding of resistant bacteria can impact the environment. We need to be mindful of these interconnected elements to ensure responsible stewardship of our health and environment.”
How can various sectors (human, animal, and environmental health) better collaborate to tackle AMR through a One Health approach?
"Now, that's a very interesting question. How can they better collaborate? For starters, we’re becoming more aware that this isn’t just a one-sector problem. First and foremost, we need to continue raising awareness that we all need to come together and talk.
The UK Research and Innovation (UKRI) recently put out a call for transdisciplinary AMR networks to tackle antimicrobial resistance. The idea is to bring people from different sectors into a network so they can start thinking about collective approaches to combating AMR. The new national action plan that the UK government published last month also emphasizes the importance of different sectors coming together and looking at various angles. This includes not just scientists, but also social scientists, engineers, and those in the creative arts.
We need people from different disciplines to sit down together and talk about the problem, and more importantly, to develop solutions to combat antimicrobial resistance. It is also important to include the public in seeking solutions to AMR. The UKRI network call is an example of a good starting point.
However, many networks that claim to be One Health AMR networks still end up working in silos. They separate into groups focused on humans, animals, the environment, and plants, with little connection between them. We need to start having discussions that don’t separate these groups but instead encourage them to work together. This is often difficult to implement, and I'm not sure how we go about this other than to try and start somewhere if we’re going to solve the problem.”
What innovations in the field of One Health are you most excited about in terms of combating antimicrobial resistance?
“Wow, that's another interesting question. There are several exciting developments, particularly the use of genomic technologies. For instance, we can examine the genomes or metagenomes of microorganisms in various One Health groupings simultaneously to understand the evolution of antimicrobial resistance. We can also analyze the genomes of different environments to see how bacteria are evolving and responding to treatment, helping us devise targeted solutions.
The advancements in sequencing and genome exploration allow us to shorten the time it takes to find solutions within different environments and ecosystems. In our lab, we use the metagenomes of various environments, especially animal gut microbiomes, to search for proteins that organisms might produce to kill pathogenic bacteria. These proteins, known as antimicrobial peptides, not only help organisms protect themselves, but are effective against many resistant microorganisms.
We leverage advances in sequencing and data analytics to identify these proteins using numerous algorithms. Some call this artificial intelligence and the use of in silico methods. We've identified many candidates with either specific or broad-spectrum activity against pathogenic bacteria and fungi. Without this approach, we would need to grow the organisms first and identify them before isolating the components with activity against pathogens, a process that would take longer.
Data analytics, sequencing advances, and the use of artificial intelligence all work hand in hand. This synergy represents a significant advance that we can certainly use.”
What do you see as the future of antimicrobial stewardship in the context of One Health, and what steps should be taken to get there?
“The future lies in bringing people together to solve the problem through cross-sector collaboration. When I say sectors, I don't just mean academic ones. We need to involve the public in the discourse on antimicrobial resistance. This includes patients who have experienced antimicrobial resistance, vets and pet owners dealing with ineffective treatments, and farmers who use chemicals and pesticides that might induce resistance.
If you compare antimicrobial resistance to something like cancer, you'll see a significant difference in public awareness. We know that a pink ribbon symbolizes breast cancer, and a red ribbon symbolizes HIV/AIDS. However, we don't have any recognizable symbols for antimicrobial resistance. This lack of awareness means that people at the grassroots level often don't understand the issue until it’s explained in detail.
We need to involve everyone in a global antimicrobial resistance campaign. This campaign should have a recognizable logo or symbol that is immediately recognizable by people, which will help to improve awareness of the problem. That's the future of stewardship—raising awareness and encouraging collective action across all sectors.
To answer the second part of the question, Dr. Oyama additionally said, “We need to start having community meetings and involve young people in the discourse. Often, the same researchers who have been doing a fantastic job studying antimicrobial resistance continue to dominate discussions in the field, but leave younger voices unheard. These younger researchers and groups are the ones who will be most affected in the future by the AMR problem and may hold the key to ‘out of the box’ solutions.
Involving as many diverse groups as possible should be our next step. This shouldn't be limited to the sciences. For example, if we want a recognizable logo, someone in the arts or creative fields might be best suited to create it. Similarly, a storyteller could effectively communicate the issue to the public, something scientists like me are obviously struggling with.
We need to leverage these diverse experiences to solve the problem. Including different perspectives and skills will help us raise awareness and develop solutions that resonate with a broader audience.”
Conclusion
A comprehensive strategy to prevent antimicrobial resistance requires the integration of One Health concepts into antimicrobial stewardship. Collaboration between the fields of environmental, animal, and human health and the involvement of divergent voices can help us develop more practical and long-lasting plans to safeguard both our health and the health of the planet. We all have a shared responsibility to combat AMR, and by using a One Health approach, we can make sure that everyone has a healthier future.
