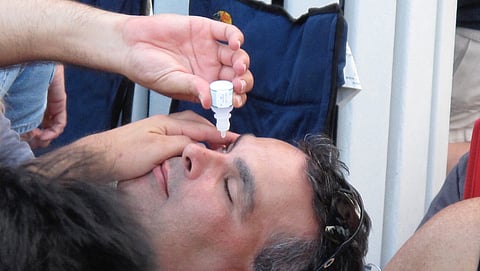

Days after granting approval, the Drug Controller General of India (DCGI) has suspended the manufacturing and marketing license for PresVu eye drops, produced by Entod Pharmaceuticals. The suspension comes in response to misleading claims made by the company, including assertions that PresVu could replace reading glasses and serve as a non-invasive solution for improving near vision. According to DCGI, the eye drop’s makers overstated its efficacy, leading to public confusion.
Entod Pharmaceuticals had claimed PresVu was the first eye drop in India that could replace reading glasses and improve near vision within 15 minutes of application. However, the active ingredient in PresVu, Pilocarpine Hydrochloride, is not a novel drug. It is commonly used and price-regulated by the Indian government. While typically used in cataract treatment, Pilocarpine Hydrochloride is also known to aid in managing presbyopia, a condition that makes it difficult for individuals to focus on nearby objects as they age. The drug works by contracting the eye's iris muscles, thus helping the eye to focus more effectively.
The DCGI’s order pointed out that Entod Pharmaceuticals failed to adequately address questions about their product and sought to justify claims for which no official approval had been granted. Although PresVu was approved for the treatment of presbyopia, the DCGI clarified that the eye drop had not been authorized to make claims about eliminating the need for reading glasses.
Entod Pharmaceuticals, however, plans to challenge the DCGI’s decision. In a statement from the company’s CEO, Nikkhil Masurkar, the firm expressed its intention to contest the order. Masurkar emphasized that the announcement of the new product was part of standard industry practice. He added that media reports about the product went viral, leading to widespread public misinterpretation. He also suggested that pharmaceutical companies frequently use broader language when describing new drugs in press releases, comparing this case to similar announcements from larger firms.
Masurkar defended the company's position by stating that, at present, no other eye drop in India is approved for the treatment of presbyopia. However, the DCGI maintained that PresVu had not been approved to reduce the need for reading glasses. Regarding the claim that the eye drop was a non-invasive alternative for enhancing near vision without glasses, Entod Pharmaceuticals pointed to a clinical trial where subjects did not wear glasses during the study.
The company also argued that one doctor had assessed the eye drop in comparison to reading glasses, leading them to claim that PresVu could enhance near vision within 15 minutes. However, the DCGI, in its suspension order, stressed that such claims had not been approved and could mislead the public.
Due to the potential for public confusion, the DCGI suspended the permission granted for the manufacture and marketing of Pilocarpine Hydrochloride Ophthalmic Solution until further notice. The regulator’s decision underscores the importance of ensuring that pharmaceutical companies provide accurate, approved information about their products, especially when the public’s health and safety are at stake.
(Input from various sources)
(Rehash/Ankur Deka/MSM)
