
Researchers at the Institute of Nano Science and Technology (INST), Mohali, have made a groundbreaking discovery in the field of catalysis, which could significantly speed up drug development and potentially lower healthcare costs. Their innovative use of catalytic droplets has led to a tenfold increase in the speed and efficiency of catalytic reactions. This discovery offers an alternative approach to traditional methods of confining molecules during catalytic processes and could pave the way for faster, more efficient chemical reactions.
For years, chemists have used physical and chemical barriers to confine molecules during catalytic reactions. While these techniques can be effective, they often come with limitations. The barriers that prevent molecules from escaping can also inhibit the movement of substrates and products, thereby slowing down the reaction itself. This challenge has long been an obstacle to improving the efficiency of chemical reactions.
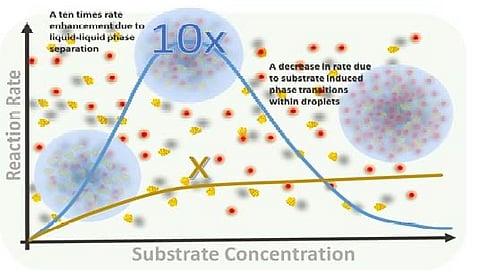
To address this issue, Professor Sarmistha Sinha and her team at INST sought to explore a new method of confining nano-catalyst molecules. Their approach involved using liquid–liquid phase separation to trap protein-metal nanocomposites inside droplets. Unlike conventional barriers, this method allows molecules within the droplets to move freely. The droplets also do not interfere with the natural structure of the proteins they contain, creating an optimal environment for catalysis.
This innovative technique resulted in a remarkable tenfold increase in catalytic efficiency. This barrier-free confinement method represents a significant advancement in catalysis, allowing for faster and more efficient reactions. The implications for industrial processes, such as drug manufacturing and energy production, are enormous.
In follow-up research, the scientists investigated how varying conditions affected the behavior of these catalytic droplets. They examined how different substrate concentrations influenced the phase and kinetics of the catalytic reaction. As the concentration of the substrate increased, the droplets, which were once fluid and dynamic, began to undergo an internal phase transition. This change restricted the movement of the substrate and the resulting products, slowing down the overall reaction rate.
This discovery highlights the importance of substrate concentration when using liquid–liquid phase separation for catalysis. While the technique has the potential to significantly enhance reaction efficiency, careful management of substrate levels within the droplets is critical to maintaining high reaction rates.
Published in the journal Nanoscale, this breakthrough represents a shift in how chemical reactions are approached. The ability to confine molecules within droplets without barriers, while simultaneously boosting reaction rates, could lead to more efficient industrial processes and technological advancements. Additionally, the understanding of phase transitions within these droplets opens the door to new technologies that capitalize on liquid–liquid phase separation, potentially revolutionizing fields ranging from drug development to sustainable energy.
Reference:
1. Rose, S. M., Silky Bedi, Sabyasachi Rakshit, and Sharmistha Sinha. “Substrate-Induced Phase Transition within Liquid Condensates Reverses the Catalytic Activity of Nanoparticles.” Nanoscale 16, no. 31 (2024): 14730–33. https://doi.org/10.1039/d4nr01402b.
(Input from various sources)
(Rehash/Ankur Deka/MSM)
